~Evaluating safety and efficacy of combination therapy with immune checkpoint inhibitors and intestinal microbiota transplantation~
Key Points
- The first clinical study in Japan to investigate the safety and efficacy of fecal microbiota transplantation (FMT) therapy followed by immune checkpoint inhibitors (ICIs) for gastrointestinal cancer patients has started for esophageal and gastric cancer patients.
- Recent studies suggest that modulating the gut microbiota may improve the response rate for patients who have received ICIs for malignant melanoma.
- Based on the results of this study, we will be examining the possibility of offering combination therapy of ICI and FMT as a new treatment option for gastrointestinal cancer.
Summary
The National Cancer Center (Chuo-ku, Tokyo; President: Hitoshi Nakagama) Hospital (Director: Yasuyuki Seto), Juntendo University (Headquarters: Bunkyo-ku, Tokyo; President: Hiroyuki Daida), and Metagen Therapeutics, Inc. (Headquarters: Tsuruoka City, Yamagata Prefecture; President and CEO: Taku Nakahara) have announced that a clinical study(Note1) on “Combination therapy of ICIs and FMT(Note2)(Note3) for gastric and esophageal cancer patients” will start in August 2024.
FMT is a medical technology in which the gut microbiota contained in the stool of a healthy person is transferred via endoscopy into the intestine of a patient to establish a balanced gut environment. FMT is expected to improve the response rate when combined with ICI therapy.
This is the first clinical study of FMT for gastrointestinal cancer patients in Japan, and will examine whether it may become a new treatment option for patients suffering from esophageal and gastric cancers treated with ICIs.
Background
Recent studies have shown that disruption of the intestinal microbiota is associated with a variety of diseases and its effect on human immune function.*1
Development of treatments focusing on the gut microbiota is progressing globally, and FMT drugs have been approved by the FDA and equivalent in the United States and Australia for the treatment of refractory Clostridium difficile infection.*2 Research on FMT in oncology*3 suggests that regulation of the gut microbiota through FMT may enhance immunity against cancer and improve the response rate of treatment for patients with malignant melanoma who have not previously responded to ICIs. Clinical studies are also being conducted in various types of cancers, including esophageal and gastric cancers.
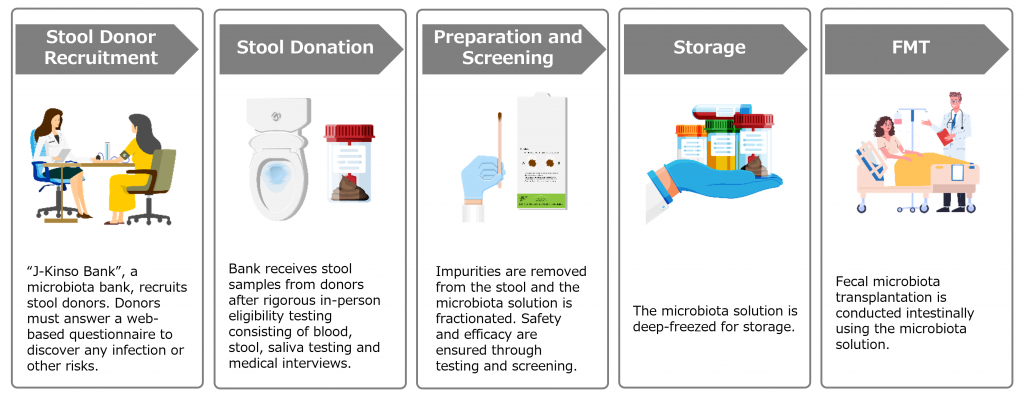
Domestically, Antibiotic Fecal Microbiota Transplantation (A-FMT) for ulcerative colitis at Juntendo University was approved in January 2023 as an Advanced Medical Care B Program, a program which facilitates patient access to novel but promising healthcare technologies whilst the government assesses toward National Health Insurance coverage. Metagen Therapeutics, as a collaborating research institute for this advanced medicine, provides, among many other things, support services related to stool donor recruitment, stool specimen management, and gut microbiota solution preparation and quality control.
This is the first clinical study in Japan to apply Juntendo University’s FMT medical technology and Metagen Therapeutics’ FMT Support Platform to the field of oncology, targeting patients with gastrointestinal cancer.
Among the various types of cancers, esophageal cancer is the 10th most common in Japan, with 25,000 diagnosed in 2020, and 11,000 succumbing to it.*4 Gastric cancer is the third most common cancer in Japan, with 110,000 newly diagnosed patients and 42,000 people dying in 2020.*4 While ICIs are allowing for more treatment options for these cancers, there is an urgent need to develop other options for patients who do not respond to ICIs. Based on the results of this study, we will examine whether this combined therapy may become a new treatment option to improve the response rate to ICIs.
Details of Initiatives
This study of “combination therapy of ICI and FMT” will be conducted in patients with unresectable advanced or recurrent esophageal and gastric cancer, to investigate the safety and efficacy. After administering three antimicrobial agents (amoxicillin, fosfomycin, and metronidazole) for one week, patients will undergo one FMT in which the gut microbiota solution is administered via colonoscopy. Treatments including ICI will be started from the day after the FMT.
FMT is a medical technique in which the gut microbiota contained in the stool of a healthy person is transferred to the intestine of a patient with a disease via endoscopy to establish a balanced gut environment.
In this study, Juntendo University and Metagen Therapeutics will collaborate in the preparation and other activities to create gut microbiota solution. The solution will be fractionated from the stool of donors who have passed the eligibility test, and safety and efficacy will be ensured through testing and screening. The solution will be prepared, frozen and stored at Juntendo University, and then transported to the National Cancer Center, where it will be stored in a frozen state until the time of the FMT.
Overview of the study
| Study title | A safety trial of antibiotic fecal microbiota transplantation for esophageal and gastric cancer patients treated with immune checkpoint inhibitors |
| Objective | Targeting esophageal and gastric cancer patients, who will undergo chemotherapy including immune checkpoint inhibitors, to evaluate the safety and efficacy of Antibiotic Fecal Microbiota Transplantation therapy. |
| Implementing Facility | National Cancer Center Hospital |
| Subject | Patients with unresectable advanced or recurrent esophageal or gastric cancers who will undergo chemotherapy including immune checkpoint inhibitors (expected number of patients: 45) |
| Method | Fecal microbiota transplantation in combination with antimicrobial agents will be performed on eligible esophageal and gastric cancer patients, followed by standard treatment for esophageal and gastric cancers, including immune checkpoint inhibitors, to evaluate safety and efficacy. |
| Implementation system | – Researcher Department of Gastrointestinal Medical Oncology, National Cancer Center Hospital Hirokazu Shoji (Principal Investigator), Ken Kato, Yuri Yoshinami (Research Secretariat) – Joint Research Institutes Dai Ishikawa, Associate Professor, Department of Internal Medicine and Gastroenterology, Juntendo University School of Medicine Metagen Therapeutics, Inc. |
Comments
Ken Kato, Chief, Department of Gastrointestinal Medical Oncology / Department of Head and Neck, Esophageal Medical Oncology, National Cancer Center Hospital

The combination therapy of intestinal FMT is a new therapy that is attracting increasing attention in the treatment of cancer. We are very pleased to start Japan’s first clinical study of intestinal microbiota transplantation therapy for gastrointestinal cancer patients at our hospital, keeping up with other countries. Through this study, we are committed to providing more effective treatment options by enhancing the efficacy of immune checkpoint inhibitors.
Dai Ishikawa, Associate Professor, Department of Internal Medicine and Gastroenterology, Juntendo University School of Medicine / CMO, Metagen Therapeutics

The number of patients in the clinical study of intestinal FMT that began in 2014 at Juntendo University has reached more than 240 cases. We are very pleased to be able to apply this technology to cancer patients. Intestinal microbiota transplantation has the potential to become a treatment option for ulcerative colitis and many other diseases in the future. We will continue our research so that we can deliver the results of our research to as many patients as possible as soon as possible.”
Taku Nakahara, President and CEO, Metagen Therapeutics

Metagen Therapeutics brings together world-class gut microbiota researchers with Ishikawa, the leader in intestinal FMT in Japan. With our knowledge of the gut microbiota, FMT (Fecal Microbiota Transplantation) support services, and FMT-driven reverse translational drug discovery(Note4) platform, we are committed to bringing new treatment options to cancer patients and their families. The company is committed to providing new treatment options to cancer patients and their families through its FMT-based Reverse Translational Drug Discovery platform. We hope that the initiation of this clinical study will lead to further research and development of gut microbiota transplantation combination therapies not only for gastrointestinal cancers, but also for other types of cancer.”
About National Cancer Center Japan
The National Cancer Center Hospital (NCCH) has led the nation’s cancer care and research since its establishment in 1962, serving its mission of “providing the best possible cancer treatments and care in partnership with communities”. We have developed comprehensive high-volume medical care provision for all type of organ cancers, and are actively advancing numerous industry sponsored trials, investigator initiated clinical trials, first-in-human (FIH) trials, and international clinical trials, all aimed at establishing and disseminating the standard of care.
About Juntendo University
Juntendo is the oldest school of Western medical education in Japan, originating from the Dutch medical school ‘Wada-juku’ established in Yagenbori, Edo (present-day Higashi-Nihonbashi,Chuo-ku,Tokyo) in 1838, during the late Edo period. Today, Juntendo University is a comprehensive health integrated university and hospital network consisting of nine faculties (Faculty of Medicine, Faculty of Health and Sports Science, Faculty of Health Care and Nursing, Faculty of Health Science and Nursing, Faculty of International Liberal Arts, Faculty of Health Science, Faculty of Medical Science, Faculty of Health Data Science, and Faculty of Pharmacy), five graduate schools, and six affiliated hospitals. Through its commitment to education, research, medical care, and liberal arts, Juntendo University aims to contribute to society and develop human resources on an international level.
About Metagen Therapeutics
The company has been accelerating its drug discovery and development work on gut microbiome-based therapeutics for various diseases such as cancer, ulcerative colitis, and Parkinson’s disease. It aims for social impact through medical care and drug discovery.
Metagen Therapeutics, Inc. is a biotech startup founded in 2020 with the mission of “Living up to the hopes of patients through microbiome science” and to create social impact through medical care and drug discovery based on gut microbiome research.
Co-founded by a Juntendo University physician and researchers from Keio University and Tokyo Institute of Technology, the company is promoting social implementation of Fecal Microbiota Transplantation (FMT) and FMT-driven reverse translational drug discovery.
Currently, the company is focusing on development in the areas of immunological diseases (inflammatory bowel disease), oncology, central nervous system diseases.
Glossary
Note 1: Difference between clinical study and clinical research
“Clinical research” refers to all research conducted on human subjects. Among these, research that affects the human body (interventional research), such as the administration of drugs, surgery, or radiation therapy, is called clinical studies. Furthermore, clinical trials, that are especially conducted with the aim of obtaining approval as a drug or medical device from the Ministry of Health, Labour and Welfare, are referred to as “clinical trials”.
Note 2: Intestinal microbiota
There are approximately 1,000 species and more than 40 trillion*5 of intestinal bacteria in the human intestinal tract, and the population of bacteria is called intestinal microbiota
Note 3: Fecal Microbiota Transplantation (FMT)
FMT is a medical technique in which the intestinal microbiota found in the stool of a healthy person is in injected into the intestine of a patient to obtain a balanced gutl microbiota.
<Reference press release>
Antibiotic Fecal Microbiota Transplantation for Ulcerative Colitis Patients Approved Under Japan’s Advanced Medical Care Program
https://www.metagentx.com/news/230104_senshinb/(linked at external site)
Note 4: Reverse translational drug discovery from FMT
It means drug discovery using gut microbiota whose safety and efficacy have been confirmed in advance by FMT. In ordinary drug discovery, drug discovery is based on translational research, which aims for practical application of drugs based on the results of basic research. FMT-driven reversed translational drug discovery enables development with a higher probability of safety and efficacy than conventional reversed translational research.
*References
1.Skelly AN, Sato Y, Kearney S, Honda K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat Rev Immunol. 2019 May;19(5):305- 323. doi: 10.1038/s41577-019-0144-5.
2.Sudarshan Paramsothy, Michael A Kamm, Nadeem O Kaakoush, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis : a randomised placebo-controlled trial. lancet. 2017 Mar 25;389(10075):1218-1228. doi: 10.1016/s0140-6736(17)30182-4
3.Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. February 4, 2021. doi: 10.1126/science.abf3363Christopher H Woelk 1, Alexandra Snyder, Modulating gut microbiota to treat cancer. 2021 February 5, 2021.
DOI: 10.1126/science.abg2904.
Bertrand Routy et al., Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial. Nat Med. 2023 Aug;29(8): 2121-2132. 2121-2132. doi: 10.1038/s41591-023-02453-x.
4.Ministry of Health, Labour and Welfare National Cancer Registry Incidence and Rates Report 2020
5.Sender, R., Fuchs, S. & Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 14, e1002533 (2016)
