Registration for donor candidates begins
(Yamagata, Japan, April 2, 2024) Metagen Therapeutics, Inc. (Headquarters: Tsuruoka City, Yamagata Prefecture; President & CEO: Taku Nakahara), a company focusing on drug discovery and development of intestinal microbiome-based therapeutics for various diseases, has started its operation of Japan’s first* intestinal microbiota bank, J-Kinso Bank. The bank has begun to recruit intestinal microbiota donor candidates (https://www.j-kinso-bank.com).
J-Kinso Bank has been established to develop medical technologies and drugs utilizing intestinal microbiota. To achieve its goal, J-Kinso Bank recruits and screens intestinal microbiota donors to collect stool. These stool of qualified donors are used in clinical trials for Fecal Microbiota Transplantation (FMT) after undergoing microbiome extraction and other processes. Donor stool will also be utilized in research and development of pharmaceuticals.
*First intestinal microbiota bank in Japan to utilize intestinal microbiota for research and development of medical technology and pharmaceuticals. According to our research (as of April 2, 2024).
Recent studies have shown that the disruption of intestinal microbiota is associated with a variety of diseases, including cancer, ulcerative colitis, Parkinson’s disease and allergies*1. Intestinal microbiota derived drugs have been approved in the United States and Australia which shows that globally, there is a growing trend to utilize intestinal microbiota in therapeutics, and much progress is being made on its research.
In Japan, Antibiotic Fecal Microbiota Transplantation (A-FMT) for ulcerative colitis was approved in January 2023 as an Advanced Medical Care B Program, a program which seeks to facilitate patient access to novel but promising healthcare technologies with governmental assessments toward National Health Insurance coverage. The verification of the efficacy and safety of FMT is currently in progress.
To implement FMT socially, and promote the development of intestinal microbiota medicine in Japan, it is essential to have a cohort of healthy donors who donate their stool.
Metagen Therapeutics began accepting registrations for intestinal microbiota donor candidates at J-Kinso Bank from April 2, 2024. Registrants will be asked to answer a web-based questionnaire (https://www.j-kinso-bank.com), and those who meet the safety criteria will be registered as intestinal microbiota donor candidates. From there, a selected number of candidates will be invited for further screening at a medical institution, after which those who pass the threshold will be invited to donate stool.
We estimated that about 10% of the registrants who do the web-based questionnaire will become stool donors *2.
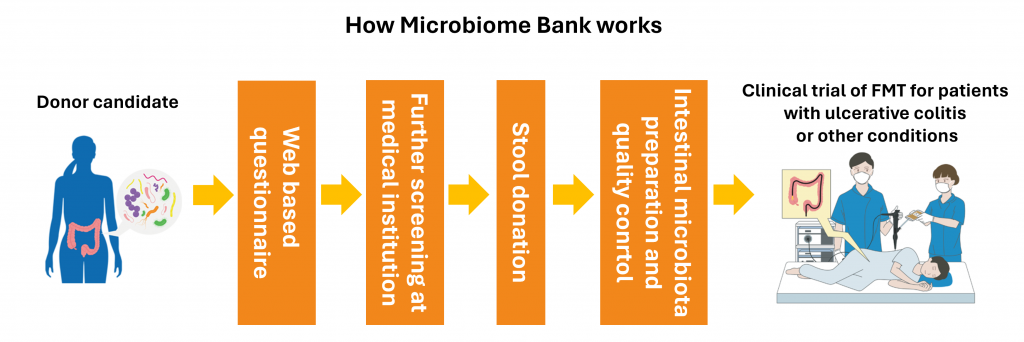
The actual collection of stool will be conducted at designated locations in Tokyo’s Juntendo University Hospital for the time being. Metagen Therapeutics plans to establish stool donation facilities in multiple locations across Japan to allow for donors across the nation to join our donation program.

Dai Ishikawa, Associate Professor of Gastroenterology, Juntendo University School of Medicine / Founder and CMO, Metagen Therapeutics, Inc.
“J-Kinso Bank is a system that connects donors to patients in need of intestinal microbiota. It is an unprecedented and new type of bank where the intestinal health of a person may be shared with many patients, leading to the treatment of diseases. Such a future is just around the corner. Juntendo University and Metagen Therapeutics have conducted a series of clinical studies to safely deliver the medical technology of FMT to patients. In the future, FMT has the potential to become not only a medical technology, but also a drug that can be transformed into a treatment option with less burden on the patients. To further advance our research and deliver the results to as many patients as possible, we need the cooperation of donors. Your cooperation is greatly appreciated”.
Metagen Therapeutics will continue to recruit donors through J-Kinso Bank to further promote the development of medical technology and drugs using intestinal microbiota. In addition, we aim to create an environment that encourages more donors to donate stools, and to realize a “gut health sharing society” in which intestinal microbiota from healthy people can be delivered to patients who need them.
What is a microbiome/microbiota?
The human intestinal tract is home to more than 40 trillion*3 gut bacteria of about 1000 species , and this population of bacteria is called microbiome or microbiota.
What is Fecal Microbiota Transplantation (FMT)?
FMT is a treatment method in which the intestinal microbiota contained in the stool of a healthy person is transplanted into the intestine of a patient to rebuild the patient’s intestinal environment. In January 2023, the Antibiotic Fecal Microbiota Transplantation (A-FMT) for ulcerative colitis was started as an Advanced Medical Care B Program. Metagen Therapeutics, a collaborating research institute for this novel but promising technology, provides platform services related to stool donor recruitment, stool specimen management, intestinal microbiota solution preparation and quality control, and other services that enable doctors to conduct FMT.
<Reference press release>
Antibiotic Fecal Microbiota Transplantation for Ulcerative Colitis Patients Approved Under Japan’s Advanced Medical Care Program
https://www.metagentx.com/en/news/230104_senshinb/
Cancer and intestinal microbiota
While immune checkpoint inhibitors(ICI) allow for more options in the treatment of cancer, new strategies are needed for patients who do not respond to ICI treatments. Recent studies*4 suggest that modulating the intestinal microbiota may improve the response rate for patients who previously did not respond to ICI.
Ulcerative colitis and intestinal microbiota
Ulcerative colitis is a designated intractable disease in Japan with the largest number of patients. The peak age of onset is in the 20s*5 and the number of patients is estimated to be more than 200,000*6. Symptoms such as abdominal pain, chronic diarrhea, and mucous and bloody stools appear and thus greatly affect the quality of life (QOL) of patients. It has been suggested that disruption of the intestinal microbiota is one of the factors contributing to the onset and exacerbation of ulcerative colitis.
Parkinson’s Disease and intestinal microbiota
Parkinson’s disease is a neurodegenerative disease of the central nervous system in which alpha-synuclein accumulates in neurons of the substantia nigra in the midbrain, causing substantia nigra cells to decrease and then dopamine to decrease. Parkinson’s disease is a designated intractable disease in Japan, and the number of patients is estimated to be about 290,000*7. As the incidence of Parkinson’s disease increases with age, the number of patients is on the rise, and it is predicted that 30 million people worldwide will be affected by 2030. Recent studies*8 have suggested a relationship between the pathogenesis of Parkinson’s disease and the intestinal microbiota, and that modulating the intestinal microbiota may improve the response rate to treatment.
About Metagen Therapeutics
The company has been accelerating its drug discovery and development work on intestinal microbiome-based therapeutics for various diseases such as cancer, ulcerative colitis, and Parkinson’s disease. It aims for social impact through medical care and drug discovery.
Metagen Therapeutics, Inc. is a biotech startup founded in 2020 with the mission of “Living up to the hopes of patients through microbiome science” and to create social impact through medical care and drug discovery based on gut microbiome research.
Co-founded by a Juntendo University physician and researchers from Keio University and Tokyo Institute of Technology, the company is promoting social implementation of Fecal Microbiota Transplantation (FMT) and FMT-driven reverse translational drug discovery. Currently, the company is focusing on development in the areas of immunological diseases (inflammatory bowel disease), oncology, central nervous system diseases, and allergic diseases.
For more information, please visit https://www.metagentx.com/en/ or follow us on LinkedIn at https://www.linkedin.com/company/metagentx/
For press inquiries, please email: pr@metagentx.com
For investor inquiries, please email: ir@metagentx.com
<Company Profile>
Metagen Therapeutics Inc.
President and CEO : Taku Nakahara, PhD
Founded: January 17, 2020
Head Office : 246-2 Minakami, Kakuganji, Tsuruoka City, Yamagata Prefecture
Tokyo Office : Toranomon Hills Business Tower 15th Floor, 1-17-1 Toranomon, Minato-ku, Tokyo
Reference:
1. Sorbara MT, Pamer EG. Microbiome-based therapeutics. Nat Rev Microbiol. 2022;20(6):365-380.
2. Bénard, M. V. et al. Challenges and costs of donor screening for fecal microbiota transplantations. PLoS One 17, e0276323 (2022)
3. Sender, R., Fuchs, S. & Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 14, e1002533 (2016)
4. Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. February 4, 2021. DOI: 10.1126/science.abf3363
5. Intractable Disease Information Center, Ulcerative Colitis ( in Japanese) https://www.nanbyou.or.jp/entry/62
6. Health and Labor Sciences Research Grants-in-Aid for Intractable Diseases, Policy Research Project on Intractable Inflammatory Bowel Disease, Summary Research Report 2016 (in Japanese)
7. 2020 Patient Survey, Injury and Disease Classification (in Japanese)
8. Nishikawa H. et al, Movement Disorder 2020 Sep;35(9):1626-1635