Microbiome Drug Discovery
Human microbiome research is emerging in drug discovery, which mainly targets the mechanism of action derived from fundamental research on the intestinal flora and bacteria themselves. Pharmaceutical product development as small-molecule drugs and probiotic preparations are to be expected.
Normally, drug discovery is carried out by translational research aiming for practical use as a drug based on the results of scientific research. The first-in-human study has shown to be a costly and timely procedure, costing millions of dollars over a period of several years.
At Metagen Therapeutics (MGTx), we are working on a novel approach to microbiome drug discovery based on Fecal Microbiota Transplantation (FMT). FMT-based drug discovery is based on ‘reverse translational research’ which focuses on the practical application of drugs, in order to proceed its development with a higher probability of safety and efficacy than usual. It aims to commercialize drugs by confirming in advance through clinical research using FMT and narrowing down the active ingredients (from different types of gut microbiota), increasing the overall success rate of microbiome drug development.
MGTx’s ‘FMT-based reverse translational drug discovery’ allows for the first-in-human trial to be implemented in the early stages of research and development, increasing the probability of success in clinical development and as a result, delivering drugs to patients in a faster and efficient manner.
Metagen Therapeutics is developing Japan’s first ‘intestinal microbiota bank’ for the social implementation of FMT and the research and development of microbiota drug discovery.
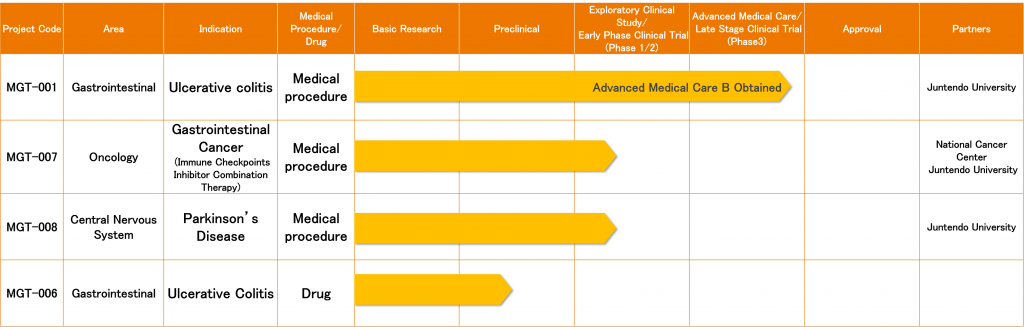
Pipeline
Clinical Research Information
Metagen Therapeutics is currently participating in the following clinical research studies as a collaborating research organization.
The clinical study information on this website is for informational purposes only and is not intended to be an advertisement or promotion of any product.
All products are currently under development and is yet to be approved.
For individual clinical research, please consult with your medical institution or health care provider. In addition, if you wish to participate in clinical research or clinical trials, please consult with your primary physician and contact the medical institution conducting the studies. Please note that research information are subject to change. For the latest information, please refer to the information published on the clinical research submission and release system (jRCT). Alternatively, you may also check with each medical institution.
Major clinical studies in which MGTx is involved as a collaborating institution
| Target Disease | Target Disease | Clinical Research Plan No. |
|---|---|---|
| Mild to moderate ulcerative colitis | Fecal microbiota transplantation in combination with antibiotics for patients with active ulcerative colitis | jRCTs031220542 |
| Unresectable advanced or recurrent esophageal cancer or gastric cancer | NCCH2308 Safety study of intestinal microbiota following combination therapy with fecal microbial transplantation and antibiotics for patients with esophageal cancer and gastric cancer treated with immune checkpoint Inhibitors | jRCTs031240170 |
| Advanced stage sporadic Parkinson’s disease | A randomized, double-blind, placebo-controlled, parallel-group study to assess the efficacy and safety of Antibiotic Fecal Microbiota Transplantation (A-FMT) in patients with Parkinson’s disease | jRCTs031240344 |